y two favorite questions from those new to the plastics industry are: How did we get so many plastics? and How do you select the best material for a given application?
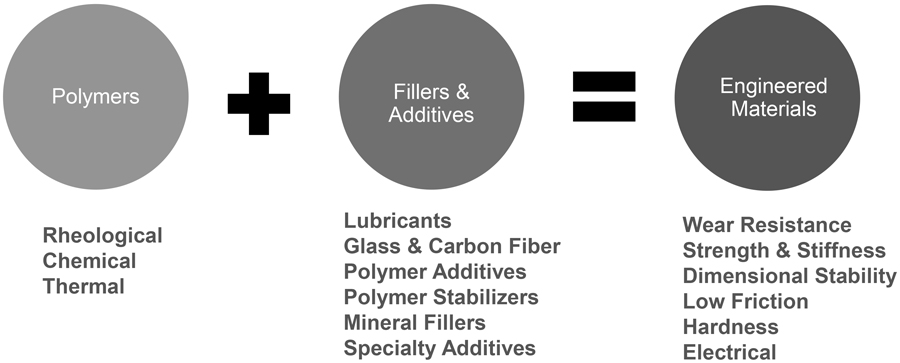
It’s impossible to answer either question without discussing the role of reinforcements, additives and blends on the properties of plastics and the influence they have on the number of material choices engineers have today. The number of polymers is finite, although trying to get performance plastics professionals to agree an actual number may be challenging. Let’s say there are less than 100 unique chemistries that we identify as manmade polymers (i.e., plastics). They include:
- Polyolefins such as polypropylene (PP) and polyethylene (PE) in all their forms and molecular weights; nylons in all their chemistries (approximately 10 varieties)
- Acetals
- Polycarbonates (PC)
- Fluoropolymers such as polytetrafluoroethylene (PTFE), polyvinyl fluoride (PVDF), ethylene-chlorotrifluorethylene (ECTFE) and fluorinated ethylene propylene (FEP)
- Polyaryletherketone (PAEK)
- Polyphenylene sulfide (PPS)
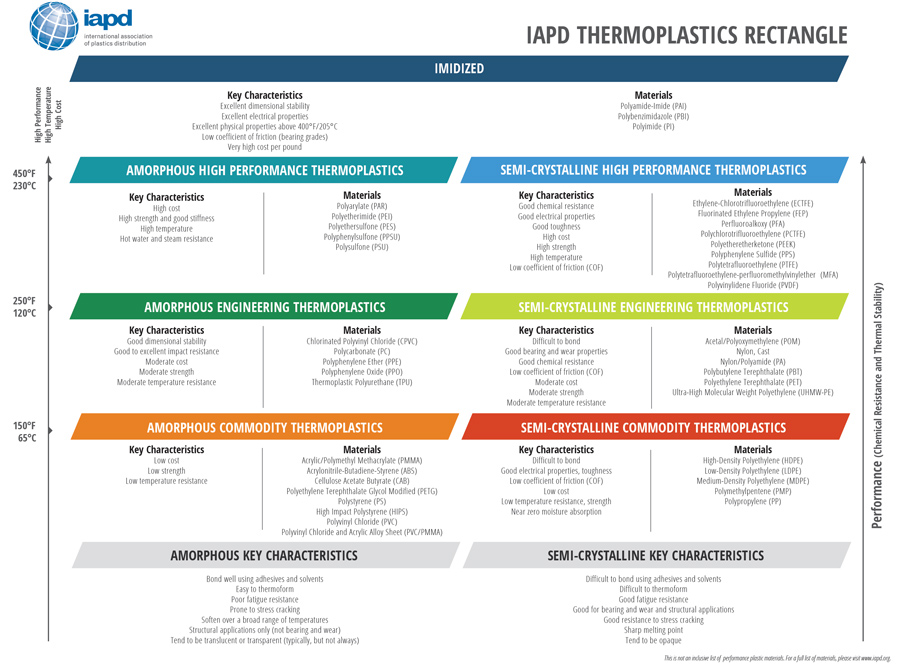
- Many others as shown in the IAPD Thermoplastics Rectangle
In the semi-finished shape world of IAPD members, the number of commercially available polymers is closer to 50.
This article highlights some of the more common ways that plastics are customized and explains how to use this information to match the materials’ potential properties more effectively with the expected performance benefits after it is in use.
Approximately 90 percent of carbon fibers are made from polyacrylonitrile (PAN) and the remaining 10 percent from petroleum pitch or rayon. Regardless of their origin, carbon fibers are conductive, meaning the electrical and thermal conductivities of the material they are added to will significantly change.
The density of glass is greater than carbon, resulting in two easily missed considerations:
- The weight of glass-reinforced plastic parts is always greater than the same size unfilled or carbon fiber-reinforced part.
- Reinforcing plastics using a weight percent means there is always more carbon fiber in a material than glass even if the percent reinforcement is the same. This means carbon fiber-reinforced materials are stronger and stiffer. They also may be more notch sensitive.
The length of both glass and carbon fibers, ranging from milled or short fibers (1 mm) to continuous lengths (including woven fabrics) influences strength, stiffness and dimensional stability. Continuous fiber composites are the strongest and stiffest of all polymers providing engineers and designer strength-to-weight ratios that far exceed most other materials including high strength metals.
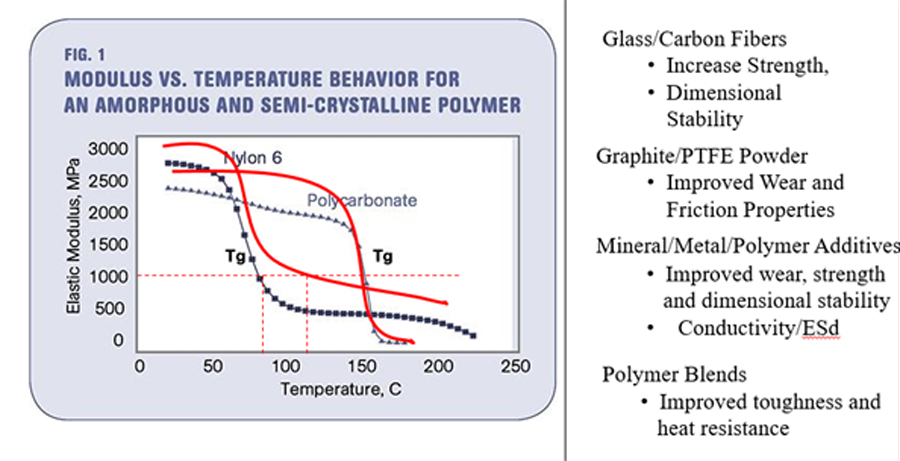
The methodology and mechanism are simple: friction leads to heat build-up, heat softens plastics and soft materials wear faster. These ingredients may work in a complimentary multi-dimensional mechanism as in the case of a typical bearing-grade polyetheretherketone (PEEK) composition (10 percent PTFE, 10 percent graphite and 10 percent carbon fiber) or the mechanism may be one dimensional. In the 10-10-10 case, the PTFE lowers the coefficient of friction while the graphite and carbon fiber increase the thermal conductivity and the strength of the material.
Wax-containing nylons use frictional heat to liquefy or melt the wax, creating boundary lubrication that helps maintain a steady state lubrication layer at the wear interface. Silicone and other liquid lubricants incorporated into materials provide boundary lubrication without the initial break-in required by solid based lubricants.
The addition of glass spheres in ultra-high molecular weight polyethylene (UHMW-PE) improves the surface hardness and abrasion resistance for bulk material handling applications. Nano-sized clays have been shown to improve the wear characteristics of UHMW-PE in high speed, wet and abrasive applications of the paper industry. The addition of mica to PTFE improves both the wear resistance and dimensional stability while maintaining the unique electrical and chemical inertness properties of PTFE. One of the early specialty PTFE processors found that by combining polyimide powders into PTFE powders, the abrasion and wear resistance of PTFE was greatly enhanced. That original recipe has been serving industrial bearing applications for 50 years. Bronze-, stainless steel- and aluminum oxide powder-filled PTFE provide strength and unique electrical properties.
An alloy/blend example may be made by combining a more chemically resistant polymer that has a lower softening temperature (Tg) with another polymer that has an inherently high Tg but limited chemical resistance. A second alloy/blend may be made by combining a lower cost more processable polymer with one that offers an attribute such as paintability or improved durability. The combination of these polymers may result in two distinct phases, each with a different Tg (blend) or a single-phase material with a single Tg that is between the two polymers (alloy). In all such combinations there is always a dominant polymer. Balanced (50:50) blends are avoided because it makes it difficult to know which polymer will be “dominant” and, therefore, predict the expected properties of the blend.
The modern era of polymer blending began in 1960, after polyphenylene-ether (PPE) was combined with styrenics. The material was commercialized in 1965. Engineering resins such as PC and acrylonitrile-butadiene-styrene (ABS) were later combined to provide automotive manufacturers a light weight, durable material for bumper covers. Combinations of polyaryletherketones such as PEEK and sulfone polymers like PES, PPSU and PSU were commercialized in the 1980s. This work expanded to include blends of polyetherimide (PEI) and polyamide-imide (PAI).